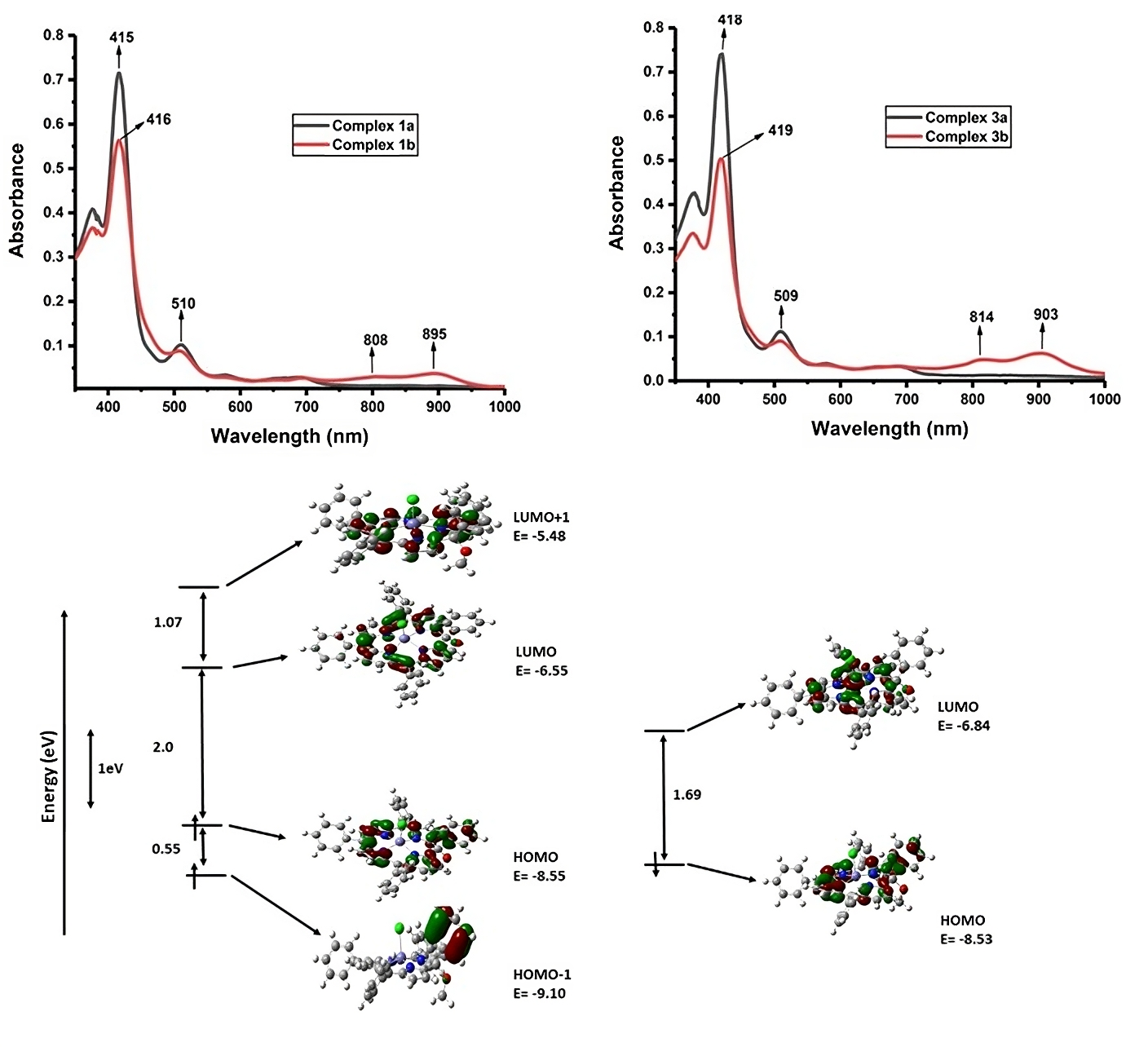
Isoporphyrin, the unique tautomer of porphyrin has been highly sought after because of its possible application in photo-medicine, as an infra-red dye and a catalyst. The electronic absorptions of isoporphyrins in the region 600 to 900 nm have made them valuable for use as sensitizers in photo-dynamic therapy (PDT) and photo-thermal therapy (PTT). The first isoporphyrin successfully prepared was in the year 1970 by Dolphin and co-workers, where zinc isoporphyrin was prepared using electrochemical oxidation of zinc tetraphenylporphyrin. In the following years, there have been multiple attempts to synthesize stable isoporphyrins. Bhuyan and co-workers have been working on the synthesis of novel isoporphyrins for diverse applications. However, the work on iron isoporphyrin is very limited even after its similarity with iron(III) oxoporphyrin radical. In the recent work, Bhuyan and co-workers at NERIST have successfully synthesized three iron porphyrins and their corresponding isoporphyrins using oxidant ceric ammonium nitrate. The low reducing potential of isoporphyrins as proved by electrochemical studies done here proves that it prompts the isoporphyrins to quickly return to their parent structure. Electrochemical studies were carried out for iron isoporphyrins for the first time. The synthesized novel complexes were also analyzed using UV-Visible spectroscopy, IR spectroscopy, ESI-Mass spectrometry, EPR spectroscopy, and 1H NMR spectroscopy. A detailed theoretical calculation was also performed to support the results. The attempt to get suitable crystals for single-crystal X-ray diffraction studies was unsuccessful due to the degenerative nature of the isoporphyrin complexes, but the author could successfully optimize the structure of a model iron(III) isoporphyrin using the DFT method. The theoretical studies corroborate the experimental electronic spectra, redox properties, and reactivity of iron isoporphyrins. This work will open new horizons to study this beautiful macrocycle for its diverse applications.
The experimental part of this work was supported by project no. YSS/2015/394, Science and Engineering Research Board, Department of Science and Technology, and computation work was supported by project no. MTR/2019/223, Science and Engineering Research Board, Department of Science and Technology. The group has already begun testing these highly reactive compounds in a variety of organic transformation processes. Dr. Bhuyan says, “I firmly believe that iron isoporphyrins have immense potential for catalyzing diverse reactions similar to iron-oxoporphyrin radical”