Histamine is a neurotransmitter and the cause of allergies but it could be an interesting ligand that can bind to metal in diverse ways. However, the coordination chemistry of histamine is not known. The parent molecule of histamine is histidine. Histidine, the parent molecule of histamine is found in the chloroplasts of photosynthesis. It has been revealed that the bulk of the bacteriochlorophyll molecules in the purple bacterial reaction centre and the purple bacterial light-harvesting complex LH2 are coordinated via their central magnesium ions to imidazole nitrogen of histidine. The structure of the photosystem II of chlorophyll-binding proteins, CP43 and CP47 have axial ligation with imidazole nitrogen of histidine coordinated via central magnesium ions. The role of histidine is exactly not known. To understand the possible role of histidine in chloroplast related to pigment degradation, Bhuyan and coworkers synthesized two histamine-bound magnesium porphyrins and found the diverse coordination modes of histamine which were never studied earlier. The crystal structure of these histamine-bound magnesium porphyrins is reported which doesn’t undergo photodegradation in the presence of oxygen and light indicating the role of axial histidine in resisting photo-degradation of chlorophyll in photosynthesis. The histamine-bound complexes also have enhanced antioxidant activity.
This work has been published in one of the top Inorganic Chemistry Journal Dalton Transactions and they have recognized the paper as a HOT article and added it to the website’s quarterly HOT article collection on 3rd October 2023. A HOT article represents the top 10% of research published in Dalton Transactions.
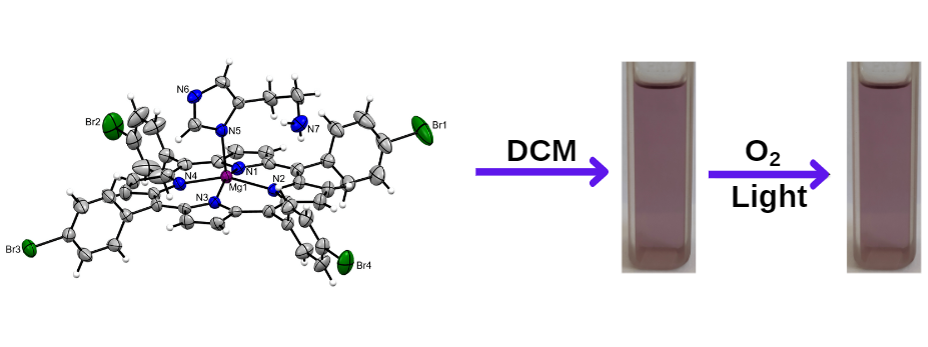
The authors studied the degradation study for magnesium porphyrin, as well as chlorophyll a extracted from the spinach in the presence of histamine. The result showed that in the presence of histamine, the photodegradation of chlorophyll stops. The crystal structure of the histamine-bound novel magnesium porphyrins [MgT(4-Cl)PP(hist)2] 1 and [MgT(4-Br)PP(hist)] 2 revealed that 1 is hexa-coordinated due to axial coordination via the nitrogen of the aliphatic amino group while 2 is penta-coordinated due to axial coordination through imidazole nitrogen. Theoretical studies support the preferable binding of compound 2 via imidazole ring nitrogen rather than nitrogen from the aliphatic amine group.
One of the major concerns hindering the use of chlorophylls in most biotechnological applications is their photo instability. Therefore, many approaches to enhance the photostability of in vitro chlorophyll have been investigated. In this work, Bhuyan and coworkers have shown that the binding of histamine to magnesium porphyrin can inhibit its photodegradation under aerobic conditions. The inhibitory role of histamine in the photodegradation of Mg-porphyrins can also be seen visually from the colour of the solutions. Authors have also performed a similar experiment for freshly isolated chlorophyll a from spinach leaves under identical conditions and found excellent expected results. Histamine causes changes in the electronic spectra of chlorophyll a that are remarkably similar to those seen with magnesium porphyrins. In the presence of histamine, Chl a did not show any spectral changes under light irradiation but chlorophyll a under light irradiation undergoes rapid degradation.
This research opens up new binding chemistry of the histamine molecule and its possible role in the stabilization of chlorophyll in vitro for technological uses.